But sugar dissolves in cold water. It just takes a bit longer. This is 9th grade chemistry. At 20°C 203.9g sugar are soluble per 100ml of water.
[Edit: Sorry, for the Americans here: At 68°F, 1 cup of sugar is soluble in 21/50 cups of water.]
Wikipedia (de): Zucker cites Hans-Albert Kurzhals: Lexikon Lebensmitteltechnik. Volume 2: L – Z. Behr, Hamburg 2003, ISBN 3-86022-973-7, p. 723.
And most of all, solubility being a function of the temperature, if you lower it the excess sugar will leave the solution and cristallize.
I came here to say this, but the best Aqua is without sugar anyway.
Have you seen how much sugar those hicks put into their tea though? It’s gotta be hot because they put coca cola grade amounts of sugar, to the point where it wont dissolve in the water anymore. Sweet tea contains 36-38 grams of sugar per 16 oz. That’s a fucking soft drink.
16 oz (454ml) can dissolve some 900 grams of sugar, far in excess of 38 grams. Sugar is ridiculously soluble in water.
As a server, southerners stare at me in wide eyed awe when I pour a disgusting amount of simple syrup into a glass of iced tea.
simple syrup
Wait, do americans use glucose syrup in kitchen?
Sugar will dissolve in unsweet tea, it’s just slower. If you can’t dissolve it in cold tea, then it wouldn’t stay in solution in hot tea that was cooled down.
For someone complaining about northerners not knowing 9th grade chemistry, it sure sounds like they weren’t paying attention themselves.
Chemistry knowledge! Sweet tea is actually a supersaturated solution. That means there more sugar in the water than could normally be held in suspension. This is achieved by heating the water so you can dissolve more solute in and then chilling it. Remember theres at least 2 diabetes worth of sugar per glass.
I highly doubt that, since any shock or impurity would cause a supersaturated solution to separate into a solution and the excess sugar.
Well, it’s the gays or atheists. Or “colored” people. Or whoever they are told to hate at that moment. This happens more than you know in this day and age:

Surprised you forgot “people who might be transgender (wE cAn AlWaYs TeLl)”
Well that explains the diabeetus.
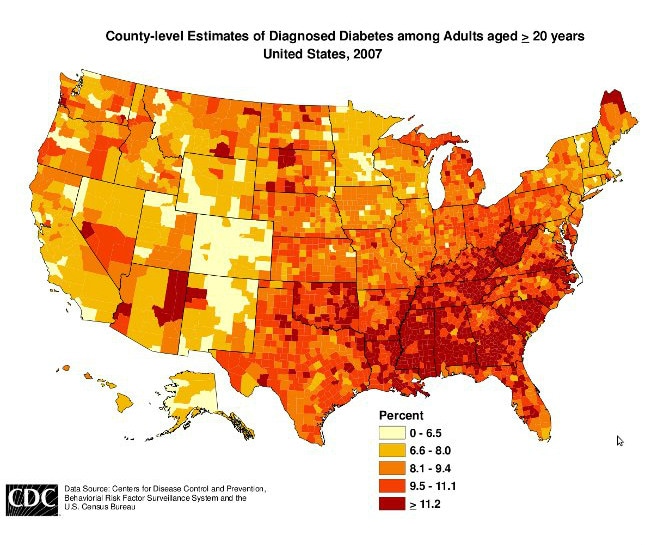
That is really just a map of poverty.




